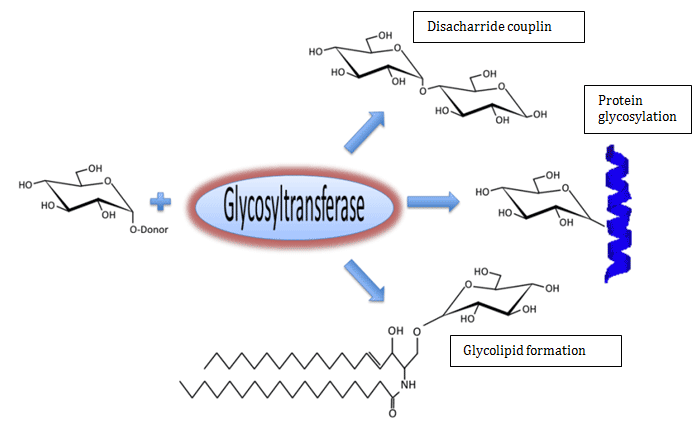
Glycosyltransferase Enzymes
Glycosyltransferase enzymes add monosaccharides onto sugars, proteins, and lipids with perfect specificity. Over half of the proteins expressed in the human genome undergo post-translational glycosylation, which is accomplished through the coordinated activity of hundreds of glycosyltransferases. Glycosylation affects a wide variety of protein functions, including activity, resistance from protease cleavage, and half-life in circulation.
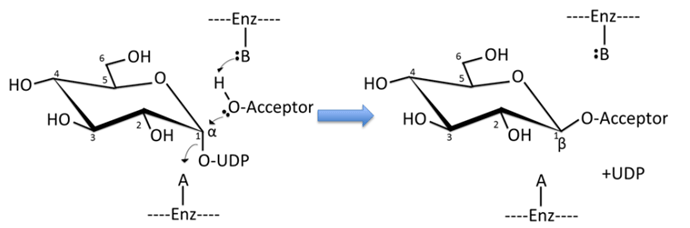
Glycosyltransferases catalyze the transfer of monosaccharides from activated donors, frequently nucleotide sugars, to acceptor substrates. Shown in the figure, glucose is transferred from UDP-glucose to an acceptor.
The half-life of protein-therapeutics in circulation can be dramatically altered by changing the glycosylation state, due to increased clearance by the kidney or hepatic receptors (Varki et al. (2003) "Essentials of Glycobiology", 2nd edition, Cold Spring Harbor Lab. Press). For example, removal of all sialic acid residues from erythropoietin (EPO) to form asialoerythropoietin decreases it's circulation half-life by more than 150 fold (Erbayraktar et al. (2003) Proc. Nat. Acad. Sci. 100, 6741).
Glycosylation can also confer resistance to protease-cleavage-inactivation of proteins. Fibroblast growth factor 23 (FGF-23) is implicated in familial tumoral calcinosis (FTC), a metabolic disorder. One of the causes of FTC is lack of O-glycosylation of FGF-23, which renders it susceptible to cleavage and unable to be secreted (Kato et al. (2006) J. Biol. Chem. 281, 18370). Low-density lipoprotein receptor is also protected from cleavage by O-glycosylation in the extracellular stalk region, and lack of glycosylation results in the loss of the receptor region (Kingsley et al. (1986) J. Biol. Chem. 102, 1576).
Glycosylation of lipids is also important for a wide variety of purposes. The glycolsphingolipid gangliosides act as intracellular signaling molecules, and glycosylphosphatidylinositol can act as membrane attachments for proteins (GPI anchor).
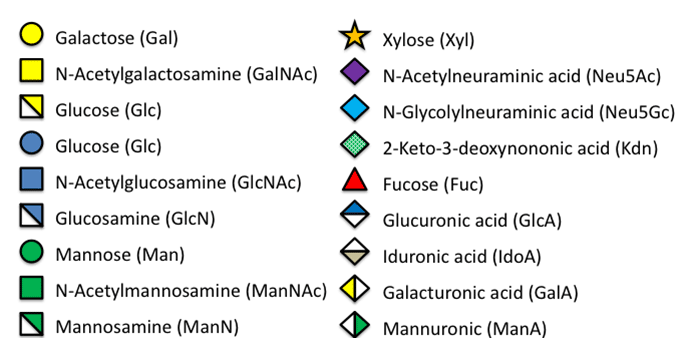
Symbolic code for common monosaccharide resins taken from"Essentials of Glycobiology." 2nd editionm Varki A, Cummings RD, Esko JD, et al., editors.
Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2009.
SBH Sciences is looking for partners to investigate our glycosyltransferase enzymes and carbohydrate antibodies as diagnostic tools, therapeutics or for production of glycol-peptide/glycol-protein products. Moreover, our extensive bioassay and in vitro biomarker analysis capabilities are available to support development of your product.
We are open for suggestions and would be pleased to hear from you.
