Antibody Development and ADCC
Expertise in Antibody Engineering & Functional Immunology
With over 15 years of dedicated experience in antibody therapeutics, we support the full spectrum of antibody-based drug discovery—from early target validation to preclinical development. Our team combines deep immunology expertise with practical experience in monoclonal antibodies (mAbs), bispecifics, ADCs, and immune cell engagers to deliver actionable, translationally relevant data.
- ADCC
- ADC
- Immunogenicity

Our Comprehensive Antibody Services
Monoclonal Antibody (mAb) Development & Characterization
Functional Assessment
-
Fc Effector Function: ADCC (NK cell-mediated killing), ADCP (macrophage phagocytosis), CDC (complement activation).
-
Neutralization assays (receptor-ligand blockade)
Developability and Safety: Immunogenicity risk assessment (T-cell epitope mapping, ADA)
Target Binding Profiling
-
Affinity measurements (flow cytometry)
-
Cross-reactivity screening
Bispecific Antibodies & Immune Cell Engagers
Mechanistic Validation
-
T-cell redirecting bispecifics (BiTEs, DARTs)
- Target dependent T-cell activation (CD69/CD25 expression, INF-ϒ release)
- Tumor cell killing (co-culture with PBMCs or engineering Jurkat reporter cells)
- Tumor Microenvironment (TME) Modulation
- Checkpoint inhibitor bispecifics (PD-1/CTLA-4, LAG-3/TIM-3)
- Cytokine antibody fusions (IL-2/IL-15 payloads)
Antibody Drug Conjugates (ADC)
Analytical Characterization: Drug-to-antibody ratio (DAR) by HIC-HPLC
Functional Potency:
-
Cell-killing mechanisms: Proliferation inhibition (MTT/XTT), apoptosis (caspase-3/7 activation).
-
Fc-mediated activity: ADCC/ADCP using engineered effector cells (Promega reporter assays).
Stability & PK/PD:
-
Serum stability (DAR decay over time).
-
Anti-drug antibody (ADA) assays for immunogenicity.
CAR-T & Cell Therapy Support
Target Engagement & Internalization: Flow cytometry (cell surface retention, antigen shedding).
Functional Potency:
-
Cytotoxicity
-
Cytokine storm profiling (IL-6, IFN-γ, TNF-α via MSD/ELISA).
Exhaustion and Persistence:
Activation/exhaustion markers (PD-1, LAG-3, TIM-3 by flow).
Metabolic profiling (Seahorse assays for oxidative phosphorylation/glycolysis).Antibody-Dependent Cellular Toxicity (ADCC) Services
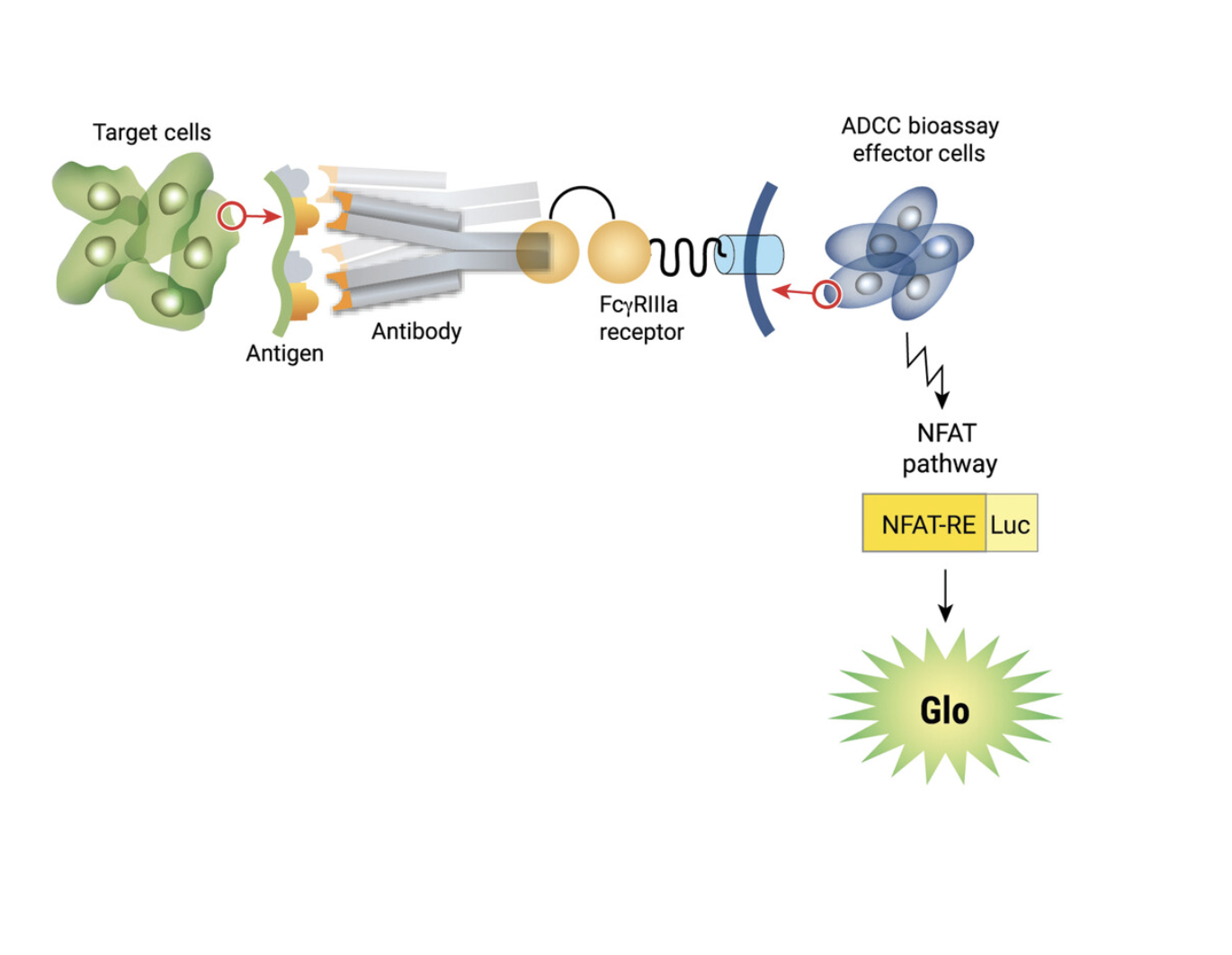
WHAT WE USE FOR THE MOST CONSISTENT RESULTS. The Promega ADCC Reporter Bioassay™ Technology
Traditional ADCC assays require donor peripheral blood mononuclear cells (PBMCs) as effector cells and typically monitor apoptosis by measuring the release of a radioactive marker. PBMC variability can lead to inconsistent results and the use of radioactive markers complicates safety.
The Promega ADCC Reporter Bioassay™ uses engineered effector cells that stably express the FcϒRIIIa receptor, coupled to luciferase expression. Activated effector cells emit luminescence in the presence of Bio-Glo™ substrate. No donor cells or radioactive markers are needed.
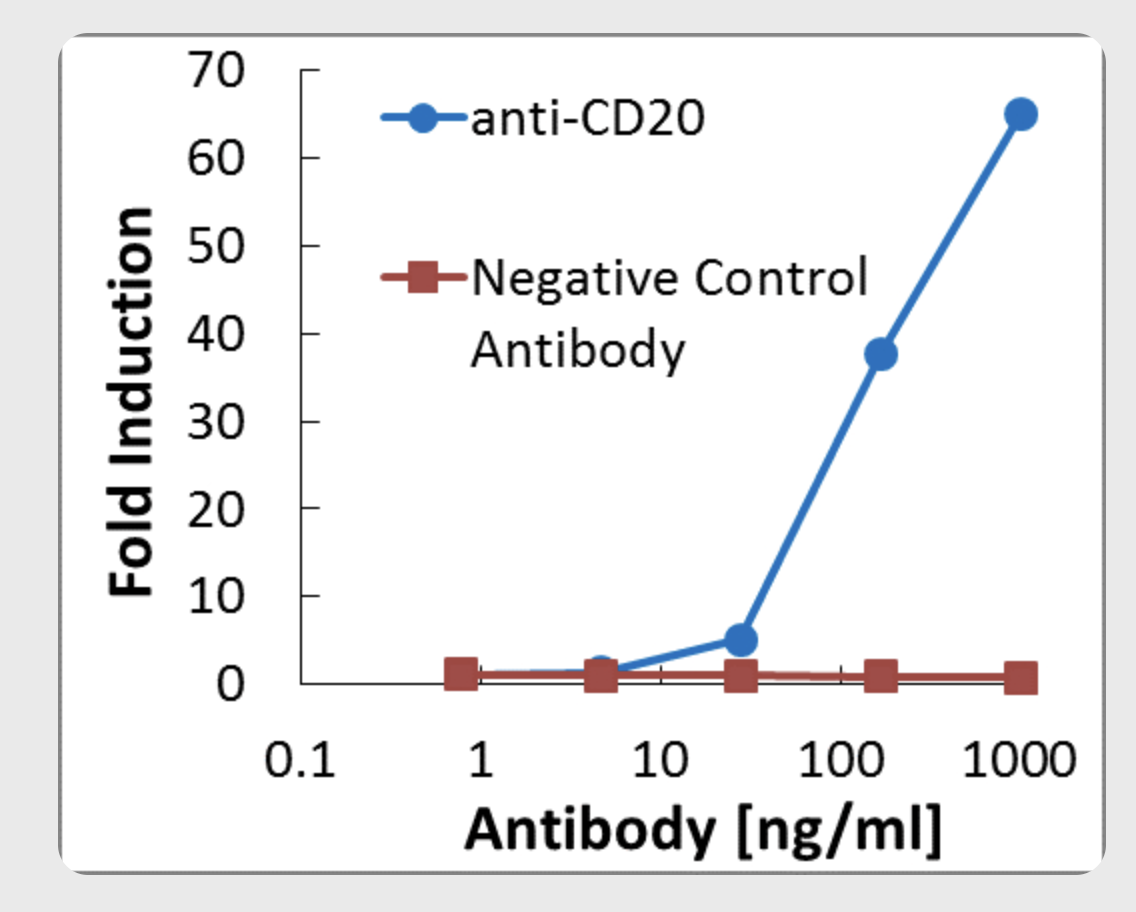
Measurement of anti-CD20 activity on WIL-2S target cells using the Promega ADCC Reporter Bioassay™
Effector cells and target cells were mixed at a ratio of 6:1 (75,000 effector cells/well) and incubated for 6 hours at 370C. Bio-Glo™ reagent was added, and luminescence was measured using a Promega GloMax Multi Detection System™. A 70 fold induction of ADCC activity was detected at 1ug/ml α-CD20.
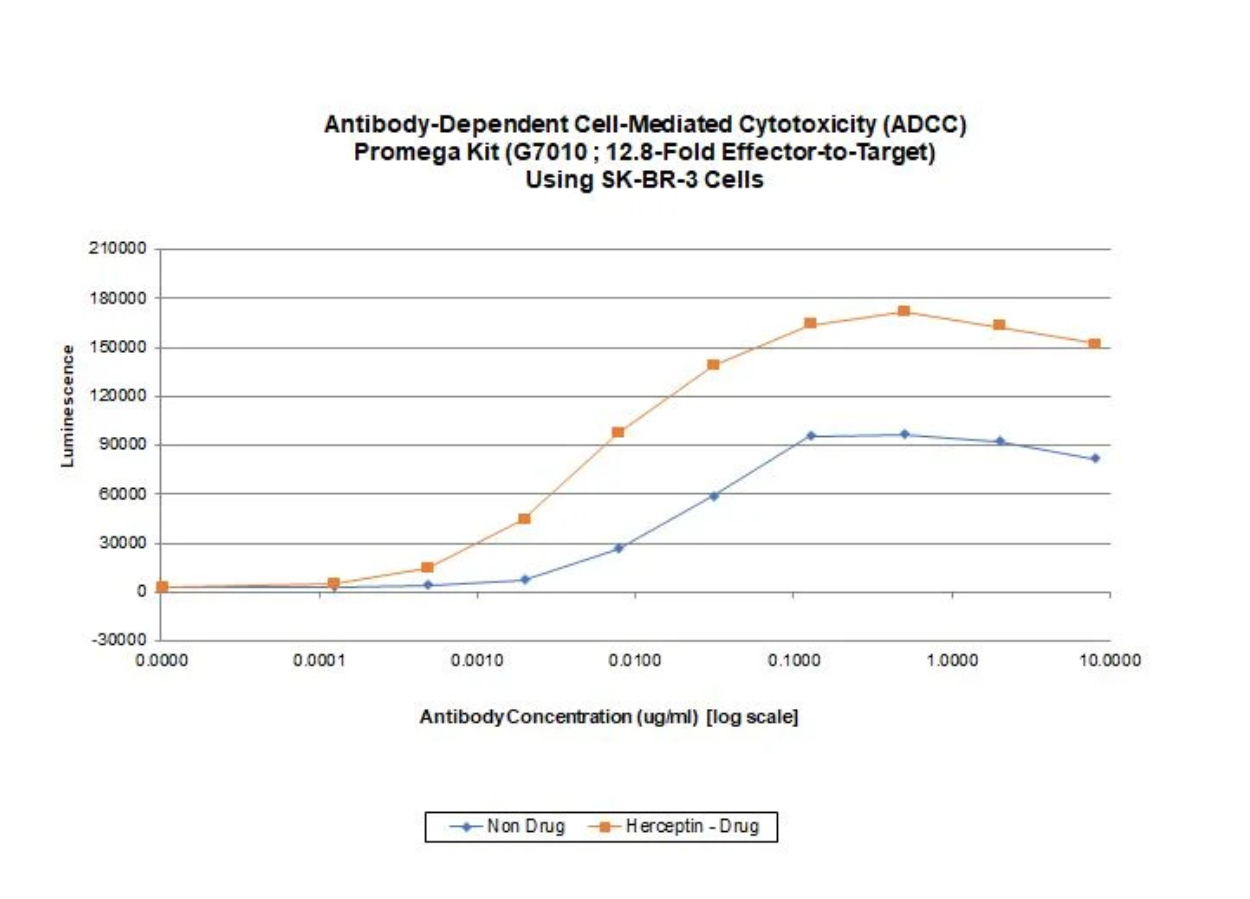
Effector cells and target cells were mixed at a ratio of 12.8:1 (64,000 effector cells/well) and incubated for 6 hours at 370C. Bio-Glo™ reagent was added, and luminescence was recorded using a CLARIOstar BMG Labtech 96-well plate reader. Herceptin-treated cells showed a 57-fold increase in luminescent signal versus control wells at 0.5 ug/ml.
Antibody Drug Conjugate (ADC) Services
ADC Services We Provide:
-
Mechanism of Action (cell proliferation, apoptosis)
-
Drug potency
-
Fc-mediated action (ADCC, Antibody-dependent phagocytosis)
-
Flow cytometry – internalization assay, cell cycle arrest assay
-
Lot Release and Stability Testing
-
Batch-to-batch comparability
-
Purity (SDS-PAGE/capillary gel electrophoresis, size-exclusion chromatography)
-
Stability (chromatographic and electrophoretic methods)
%20Analytical%20Characterization%20Drug-to-antibody%20ratio%20(DAR)%20by%20HIC-HPLC%20Functional%20Potency%20Cell-killing%20mechanisms%20Proliferation%20inhibition%20(MTTXTT)%2c%20apoptosis%20(caspas.png?width=600&height=503&name=Antibody-Drug%20Conjugates%20(ADCs)%20Analytical%20Characterization%20Drug-to-antibody%20ratio%20(DAR)%20by%20HIC-HPLC%20Functional%20Potency%20Cell-killing%20mechanisms%20Proliferation%20inhibition%20(MTTXTT)%2c%20apoptosis%20(caspas.png)
ADC Analysis with Ligand-Binding Assays
Ligand Binding Assays (ELISA) are ideally suited for the bioanalysis of ADCs and their component parts (except for free drug). For pre-clinical or clinical sample (plasma or serum) analysis, we can provide:
-
PK/TK studies for ADCs
-
Immunogenicity assessment
-
Biomarkers
-
Long term stability
Immunogenicity Assessment
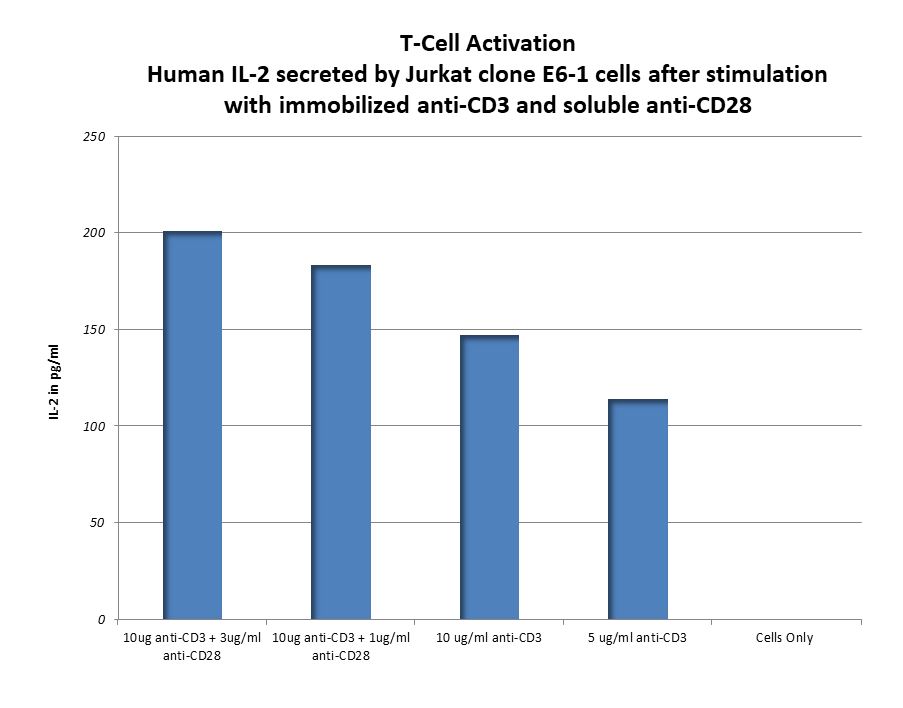
T-Cell Based Immunogenicity Assays
This assay measures human T-cell activation following exposure to a potential drug. For this assay SBH uses PBMC from StemExpress, isolating the T-cells through negative selection using STEMCELL EasyStep Human T-Cell Enrichment Kit. In addition to using T-cells isolated from PBMCs, SBH has also used Jurkat E6-1 cells.
Activation markers such as CD69, CD25, CD4, and CD8 are analyzed using multiple platforms, including ELISA, Luminex and flow cytometry. See the data below of T-Cell Activation by CD3 and CD23 antibodies.
Services for Every Stage of Drug Development
In-Vitro Models
Designed to designed to predict the potential immunogenicity of proteins, antibodies, and other novel biologics, helping to anticipate how a new therapeutic may interact with the immune system.
Immune Response Evaluation
Conducted in pre-clinical models as well as clinical samples to assess how the immune system responds to a particular therapeutic, which is critical in understanding the safety and efficacy of biologics before moving into clinical trials.
